Biomaterials: An Overview .... continued
by Tim B Hunter, MD, MSc and Pablo Gurman, MD
Fluorocarbon Polymers
Polytetrafluoroethylene (PTFE), commonly known as Teflon (DuPont), is the most recognized fluorocarbon polymer (fluoropolymer). Fluoropolymers have multiple strong carbon fluorine bonds with high resistance to solvents, acids, and bases. PTFE's repeating unit is similar to that of polyethylene except the hydrogen atoms are replaced by fluorine atoms. PTFE is made from tetrafluoroethylene gas placed under pressure with a peroxide catalyst in the presence of excess water for removal of heat (Park, 1994). Fluorocarbons in general are notable for their non-sticking and friction reducing properties.
High Performance (High Temperature) Thermoplastics
High performance (high temperature) thermoplastics polymers are designed to match the properties of light metals. These polymers have stiffened main backbone chains which give them excellent mechanical, thermal, and chemical properties. These are considered high performance plastics which differ from standard plastics by their increased temperature and chemical stability. They are produced in smaller amounts than standard plastics and are more costly.
A common distinction among polymers is whether they are thermoplastic or thermoset. Thermosets can only be shaped once, while thermoplastics can be reheated and remolded several times. High-performance plastics are always thermoplastic. Thermoplastics can be divided into three categories: commodity thermoplastics, engineering thermoplastics (ETP), and high performance thermoplastics (HPTP). High performance thermoplastics are also known as high temperature thermoplastics. They have high melting points between 6500 and 7250 K and high molecular weights greater than 20,000 g/mol.
High performance thermoplastics are found in a diverse range of industries - electronic, biomedical, automotive, aerospace, telecommunications, and environmental monitoring. They display an excellent resistance to most chemicals and to water over wide temperature ranges. Poly ether ether ketone (PEEK) is a noteworthy high temperature thermoplastic. It is usually manufactured with fiberglass or carbon reinforcement and has many biomedical applications, such as prosthetic intervertebral disks.
Rubbers
Rubber is defined by ASTM as "a material which at room temperature can be stretched repeatedly to at least twice its original length and upon release of the stress, returns immediately with force to its approximate original length." Rubber is the basic constituent in tires of all types, and more than half of all rubber produced goes into automobile tires. Natural rubber is quite important commercially, but more synthetic rubber is used commercially than natural rubber.
The main chemical constituents in rubbers are "elastic polymers" (elastomers), which are large chainlike molecules. They can be stretched to great lengths and recover. Natural rubber consists of solids suspended in a milky fluid (latex) which circulates in the inner portions of the bark of certain tropical and subtropical trees and shrubs.
Natural rubber is made predominantly from the latex of the Hevea brasiliensis tree. Its chemical formula is the same as that of polyisoprene. Natural rubber is biocompatible with blood. Natural rubber treated by x-rays and organic peroxides produces a rubber with superior blood compatibility compared with rubbers made by conventional sulfur vulcanization.
Rubber's repeated stretchability is attributable to the cross-links between chains that hold the chains together. The amount of cross-linking in natural rubber controls the flexibility of the rubber. The addition of 2% to 3% sulfur results in a flexible rubber. The adding as much as 30% sulfur makes it a hard rubber. Antioxidants can be added to rubbers to protect them against decomposition by oxidation, giving them improved aging properties. Fillers such as carbon black or silica powders may be added to rubbers to improve their physical properties (Park, 1994).
Synthetic rubbers were developed to substitute for natural rubber and are used widely in everyday products. The Natta and Ziegler types of stereospecific polymerization techniques have made the development of synthetic rubbers possible. Silicone rubber, developed by Dow Corning, has considerable medical use in tubes, catheters, drains, and sometimes in artificial joints. The repeating unit in silicone rubber is dimethyl siloxane
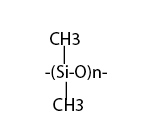
which undergoes condensation polymerization. Silastic rubber is a trademark product of Dow Corning for a specific soft, flexible silicone rubber. Medicalgrade silicone rubbers sometimes use stannous octate as a catalyst which can be mixed with the base polymer at the time of implant fabrication. Silicone rubbers also use silica (SiO2) powder as fillers to improve their mechanical properties. The more fillers that are used, the higher the density and the harder the resulting rubber (Park, 1994).
Back to Top
CERAMICS
Ceramics are inorganic compounds with favorable properties for industrial and medical uses. Ceramics include silicates, metallic oxides, carbides, and various refractory hydrides, sulfides, and nitrides. Some ceramics derived from oxides, such as Al2O3, MgO, and SiO2 contain both metallic and nonmetallic elements. Other ceramics are ionic salts: NaCl, CsCl, and ZnS.
While ceramics are considered to be "inorganic," the "carbons" (diamond and carbonaceous structures like graphite and pyrolized carbons)
are ceramics, even though they are "organic" because of their carbon content. Carbons have been used for artificial heart valve disks, percutaneous buttons, and dental implants. Although their black color can be a drawback in dental applications, carbons, in general, are desirable due to good biocompatibility and ease of component fabrication.
Ceramics get their attention
as candidates for implant materials, because they are inert to body fluids and have high compressive strength and a good aesthetic appearance. Ceramics are usually quite hard; in fact, the measurement of hardness is calibrated against ceramic materials. Diamond is the hardest ceramic with a score of 10 on Mohs Hardness Scale and talc (Mg3Si4O10[OH]2) is the softest with a score of 1. Others notable ceramics have Mobs' scores in between - alumina (Al2O3; score 9), quartz (SiO2;
score 8), and apatite (Ca5P3O12F; score 5).
Ceramic materials have high melting temperatures because of their high
bonding energy.
Metals and polymers are not difficult to shear. On the other hand, ceramics are difficult to shear because of the ionic nature of their bonding. For shear to take place, planes of atoms must slip past each other, but in ceramics ions with the same electric charge repel each other, making moving the planes of atoms very difficult. Ceramics are therefore non-ductile, and creep at room temperature is almost nonexistent.
Ceramics are very sensitive to notching and microcracks. Instead of undergoing plastic deformation (or yield), they fracture elastically once a crack propagates giving them low tensile strength compared to their compressive strength. In compression, any cracks or pores tend to be closed. In tension the opposite is true. In the case of compression, the cross-sectional area of the narrowest section does not decrease. In tension the cross-sectional area of the narrowest section becomes smaller. Since the area is made smaller by the applied force, the actual stress becomes larger. This worsens the stress-concentration effect, which is a much more important factor than the change in cross-sectional area. However, If a ceramic is made flawless, it becomes very strong even in tension. Glass fibers made this way have tensile strengths twice that of steel (Park, 1994).
Aluminum Oxides
High-purity alumina (aluminum oxide, Al2O3) comes from natural bauxite (a mixture of aluminum oxides) and native corundum. Corundum is a crystalline form of aluminum oxide containing traces of iron, titanium, vanadium, and chromium. It is a naturally transparent material with different colors depending on the impurities present. Rubies and sapphires are forms of corundum.
Sapphire and ruby have been used successfully to make implants. One can make large single crystals by feeding fine alumina powders onto the surface of a seed crystal, which is then slowly withdrawn from an electric arc or oxy-hydrogen flame as the fused powder builds up.
Alumina is usually quite hard and is used for abrasives and bearings for watch movements - the hardness is accompanied by low friction and wear. These are major advantages for using alumina as a joint replacement material, but alumina does have the disadvantage of being brittle.
Hydroxyapatite
Apatite is a group of phosphate minerals. The mineral part of bone and teeth consists of apatite of calcium and phosphate (hydroxyapatite) which has the chemical structure Ca10(PO4)6(OH)2. If there is substitution of OH with F, hydroxyapatite has a greater chemical stability, probably one of the reasons for the better caries resistance of teeth by fluoridation. Hydroxyapatite has excellent biocompatibility, and it appears to form a direct chemical bonding in the body with hard tissues.
Hydroxyapatite can be used as artificial bone. It is an important biomaterial for orthopedic practice both in a solid and in a porous form and as a coating on implants (see Bone Grafts and Bone Substitutes).
Glass-Ceramics
Glass-ceramics are polycrystalline ceramics made by controlled crystallization of glasses developed by S.D. Stookey (1915-2014) of Corning Glass Works in the early 1960s. Ceramic glasses were first used in photosensitive glasses in which small amounts of copper, silver, and gold are precipitated by ultraviolet irradiation darkening the glass. Bioglass is a well known glass ceramic developed for medical devices.
The main drawback of glass-ceramics like other glasses and ceramics is their brittleness. Their use in orthopedics is restricted, because only certain ones of them stimulate bone formation and are biocompatible. They can't be used for making major load-bearing devices, and it is also doubtful their direct bonding with hard tissues can be maintained over a long time. Old cells are replaced by new ones, constantly destroying any initial bonding between the glass-ceramic and the body tissues. Glass-ceramics are successfully used as fillers for bone cement, as dental restorative composites, and as coating material.
Other Ceramics
Other ceramic materials are constantly being examined for industrial and medical uses. Some of these ceramics are titanium oxide (TiO2), barium titanate (BaTiO2), tricalcium phosphate (Ca3[PO4]2), and calcium aluminate (CaO·Al2O3).
Carbons
Carbons are the only "organic" ceramic materials. Carbons come in many forms - allotropic, crystalline diamond, graphite, noncrystalline glassy, and quasi-crystalline pyrolytic carbon. Pyrolytic carbon (pyrocarbon) is widely utilized for implant fabrication. Because blood clots do not easily form on it, pyrocarbon is used for artificial heart valves and for coatings on orthopedic implants.
Composites
Composites are made from one or more materials which have different physical or chemical properties. The resultant composite material has characteristics different from its individual components. However, the individual components remain separate and distinct within the composite material. Common everyday composites are mortar and concrete.
With respect to biomaterials, polymer composites are sometimes designed to increase polymer stiffness, strength, and fatigue life. Carbon fibers have been incorporated in carbons themselves and into plastics. Ultrahigh molecular weight polyethylenes may be converted into composites for potentially improving implant wear resistance and longevity. Inclusion of bone particles in PMMA cement somewhat improves its stiffness and considerably improves its fatigue life. The bone particles at the interface with the patient's bone are ultimately resorbed and are replaced by ingrowing bone tissue which increases the implant fixation.
Composites are most commonly used in dental restorations and in dental cements. Composite materials are also used for prosthetic limbs because of these materials' low density/weight and high strength (AZO materials).
Back to Top
Biomaterials - other considerations
Surface Properties
The surface property of a biomaterial is probably its most important parameter. When an implant or device is placed into living tissue, the surface chemistry determines how the implant biomaterial interacts with body tissues and fluids. Surfaces of metallic implants can corrode releasing metallic ions into solution. These may cause minimal effects, but they can lead to severe inflammation with particle disease and eventual implant failure.
Inorganic glasses and clays may undergo ion-exchange with body tissues and fluids. Polymers don't corrode per se but can leach monomers or other materials. Because of these considerations, implant surface perfection is important. There are a number of ways to treat an implant surface - chemical bath, electric current application, grinding, and polishing (Slide Share: Biomaterials-Unit 1). Grinding removes surface impurities, and polishing further smooths the surface.
Biomaterial Treatment and Sterilization
The toughness of some biomaterials (metals and glasses) is enhanced by annealing. The material is heated to below its melting temperature for a set time which is then followed by a usually slow controlled cooling. On the other hand, an alloy can be heated and then rapidly cooled (quenching) to alter its properties favorably.
A most important consideration is the proper sterilization of an implant. Sterilization failure not only subjects a patient to an infected implant with certain implant failure, but it also subjects the patient to possible abscess formation or general septicemia and death. Classical sterilization procedures include steam, ethylene oxide treatment, or gamma irradiation. Other methods include microwave sterilization and use of other chemical sterilants. These methods if performed using accepted standards, achieve high sterility levels, but, in many cases, they may compromise the physiochemical properties of a material and effect its biocompatibility.
This challenge of sterilizing certain biomaterials is somewhat similar to the problem of sterilization of planetary space probes to prevent forward contamination of a planetary body with earth organisms (Hunter, Space Quarantine). Vigorous heating of delicate electronics in a space probe may disrupt circuits and destroy the functionality of the probe. The probe may be heated enough to kill most bacteria but to a low enough temperature to spare the electronics and other parts of the probe, especially in cases where the probe is unlikely to actually contact a planetary body or is visiting a body very unlikely to harbor any form of life.
Such a compromise cannot usually be made for medical devices. Anything inserted into living tissues must achieve high sterility assurance levels (SAL). This often requires the development of sterilization methods beyond those traditionally used. For example, a dense carbon dioxide based technique can be used to sterilize soft polytethylene glycol (PEG) hydrogels. Oxygen gas and gaseous forms of hydrogen peroxide are sometimes used for sterilization. Many medical devices present significant challenges for maintaining device integrity while insuring proper sterilization.
The infection risk level of a medical device can be assessed according to its use (Migonney, 2014):
Contact with blood or vascular system - high risk
Contact with mucous membranes or skin wound - medium risk
Contact with healthy skin - low risk
Washings and cleanings have to precede device sterilization. In fact, a material that has not be thoroughly washed and cleaned cannot be safely sterilized. Decontamination means bacteriostatic action has been applied to an object. Disinfection means bactericidal processing has been applied where disinfectant products are used. Disinfection produces destruction of dangerous germs. Sterilization is the killing of all microorganisms so that the product is sterile (Migonney, 2014). Whatever method is used for sterilization, manufacturers of medical devices have to ensure their products are safe for human use. This not only includes the device works as intended, but also that it has a very low likelihood (less than one in a million) of introducing microorganisms into the body.
Back to Top
Biomaterials testing
Biomaterials must be tested to establish their usefulness and safety. These tests can be broadly divided into in vitro tests and in vivo tests.
In vitro testing
In vitro testing includes cell culture testing, tissue culture testing, and organ culture testing. In cell culture testing the biomaterial is exposed to a cell mono-layer either by direct contact of the cell culture to the bulk biomaterial or diffusionally through introduction of biomaterial material extract or particles to the cell culture media. Tissue culture testing involves the use of portions of an intact tissue without prior cellular dissociation. Organ culture testing involves the culture of isolated organs with the biomaterial in vitro.
Some of the disadvantages of in vitro testing of biomaterials include:
- Lack of local blood circulation and lymphatic drainage (cells can only interchange nutrients by diffusion)
- Lower metabolic rate
- Lack of adequate micro-environment (for chemistry and topography)
- Some tissues may require mechanical stimuli (muscle and cardiac tissues)
Considerations when performing in vitro testing for biomaterials include but are not limited to:
- Proper selection of cell type
- Proper selection of dose - some cells are tolerant of biomaterials only at specific concentrations. In addition, these concentrations might not be representative of those found with usage of the final human product.
In vivo testing
In vivo testing of biomaterials is divided into two main types:
- Animal studies
- Human studies, also known as clinical trials
Animal studies:
Animals are used in medicine for two purposes: a) for developing disease pathogenesis models in selected animals (i.e., animal models); b) as test subjects for establishing the safety and efficacy of drugs, vaccines, and medical devices.
Each animal species has unique features that makes it favorable for testing specific biomaterials and implants. For example, the mouse is very widely used due its relatively low cost, short gestation time, ready availability, and low inter-individual variability. The rabbit is an excellent animal model for ophthalmology due to the relatively large size and similarity of the rabbit eye with the human eye. The pig is very much used due to its similar hemodynamics, immune system, physiology, and disease progression compared to the human.
In vivo animal studies of biomaterials can be classified as non-functional or functional. Non-functional testing involves having materials "floating” passively in the animal tissue. The advantages of non-functional testing are simplicity and the direct interaction of the material with the chemical and biological environment of the animal body. The disadvantages of non-functional testing are the absence of any functional elements, which somewhat limits the utility of the testing. Selected anatomical sites for implantation of biomaterials in non-functional animal testing include the subcutaneous tissues and the peritoneal cavity due to the simplicity of the surgical procedure for implantation.
In functional animal testing, the biomaterial must be placed in a functional mode similar to that it would experience in a functional human implant. In this case, the anatomic site for implantation has to correspond to a similar location in a human. The main limitation for functional animal testing of implants is the design, fabrication, and testing of a completely functional animal version of an implant can be very difficult. In addition, understanding the performance of an implant used in a animal can be quite challenging to assess due to inherent difficulties when dealing with animals, because animals cannot talk and cannot directly describe pain or discomfort.
The ethical and legal considerations involved in scientific experimentation using animals are many and complex. Anyone involved in animal experimentation must have a thorough understanding and training in these issues. It is important to note extended discussion about animal testing from many points of view can be found at multiple sources, including university and governmental sites as well as many medical, veterinary, and non-governmental organization sites. A good overview of animal testing is found at the U.S. Food and Drug Administration (FDA) website: Why are animals used for testing medical products.
Clinical trials:
Clinical trials will not be discussed here. The legal, regulatory, and quality assurance issues for the development, testing, and marketing of drugs and medical devices for clinical use are more extensively discussed in the essay Medical Devices: legal, regulatory, and quality assurance considerations.
Back to Top
|