Biomaterials: An Overview .... continued
by Tim B Hunter, MD, MSc and Pablo Gurman, MD
POLYMERS
Polymers (poly= 'many'; mer= 'part') are large molecules which are composed of repeating individual molecular units known as monomers linked together. The linkage is by primary covalent bonding in the main backbone chain by means of C, N, O, and Si atoms. Polyethylene, which is made from repeating units of ethylene (CH2=CH2 ), is the simplest polymer. Polyethylene is widely used for biomedical applications including weight-bearing surfaces for joint prostheses. In polymers the carbon atoms share electrons with two other hydrogen and carbon atoms: -CH2--(CH2-CH2 )n-CH2-, where n indicates a number of repeating units in an average length chain (Park, 1994).
At low molecular weights polymers behave as a gas or as an oil. At somewhat higher molecular weights they behave as a wax like paraffin wax used for candles. For a polymer to form a strong solid material it has to be a giant molecule. The repeating unit, n, should be well over 1000. This means the molecular weight (MW) of the polymer is over 28,000 grams per mole. The main backbone chain of a polymer does not have to be composed of carbon. It can be of entirely different atoms. The polydimethyl siloxane (silicone rubber) has silicone (Si) and oxygen (O) as its backbone atoms: -Si(CH3)|[O-Si(CH3)2|n-O- (Park, 1994).
There are potentially an infinite number of polymers. The polymer molecular size can be varied as well as differing atoms being used for the main backbone chain. In addition, the side-group atoms can be changed. If the side group atoms of hydrogen in polyethylene are substituted with fluorine (F), the resulting material is Teflon (polytetrafluoroethylene).
To force small molecules (monomers) into linking and forming a larger polymer, one has to make the monomers lose electrons by the processes of condensation and addition. This done by controlling the reaction temperature, the pressure, and the presence of a catalyst(s) to which one subjects the monomers. These factors then control the degree to which the monomers are put together into a chain (Park, 1994).
There are both natural and synthetic polymers. Polysaccharides and proteins are naturally occurring polymers. Natural polymers are often recognized by cells and are catalysts for many critical functions in tissues. Natural polymers usually have poor mechanical properties. Polysaccharides and proteins are made by condensation polymerization with the condensing molecule of natural polymers always being water (H2O). Natural polymers may have strong immunogenicity, and they can be unpredictable with lot-to-lot variation.
Synthetic polymers are designed for industrial and biomedical applications. The first commercial polymer products were introduced in the 1930's with the polyamide nylon made by condensation polymerization. Other synthetic condensation polymers are polyester (Dacron), polyurethane, and polydimethylsiloxane (Silastic elastomer). Synthetic polymers have unpredictable biocompatibility, which must be tested. However, they can be made with minimal lot-to-lot variation, and they have a "tunable" ability for being designed with specific features.
Condensation polymerization represents the formation of a polymer involving the loss of a small molecule (the condensing molecule) when the two parent molecules combine. Addition polymerization is achieved by rearranging the bond within each monomer. Each "mer" has to share at least two covalent electrons with other mers, and the monomers have to have at least one double or triple bond.
Addition polymerization is used for vinyl monomers to produce important vinyl polymers. Table 2 shows some of the commercially important vinyl monomers used for addition polymerization. Vinyl polymers have the following forms:
 ...or ... ...or ...
Table 2 Monomers for Addition Polymerization (from Park, 1994)
| Monomer Name |
Chemical Formula |
| |
|
| Vinyl chloride |
CH2=CHCl
|
| Vinyl acetate |
CH3COOCH=CH2
|
| Styrene |
CH2=CH-C6H5
|
| Vinylidene chloride |
CH2=CC12
|
| Methyl acrylate |
CH2=CH-COOCH3
|
| Methyl methacrylate |

|
| Acrylonitrile |
CH2=CH-CN
|
Polymers are easily fabricated into many forms - fibers, textiles, films, gels, oils, sols, and solids. This means they have a great many industrial and biomedical applications. Many synthetic polymers bear a close resemblance to natural tissue components, such as collagen. This allows direct bonding with natural substances, permitting adhesive polymers for closing wounds or gluing orthopedic implants in place.
Back to Top
Polyolefins (Polyethylene and Polypropylene)
Polyolefins are linear thermoplastics that can be remelted and reused. Polyethylene and polypropylene are important polyolefins. The annual global production of polyethylene is at least 80 million tons. It is a very important commercial plastic and is available in three major grades: low density, high density, and ultrahigh molecular weight (UHMWPE). Ultrahigh molecular weight polyethylene has a molecular weight > 2 x 106 g/mol. It has many applications in orthopedics, especially for load-bearing surfaces such as total hip and knee joint arthroplasties.
Polyethylene is a vinyl polymer having the structure shown below with R= H. Polyethylene has no known solvent at room temperature. Only high temperature and pressure sintering of polyethylene can be
used to produce desired products as conventional extrusion or molding processes are difficult to use (Park, 1994).

Another olefin polymer is polypropylene (also known as polypropene) with R=CH3. Polypropylene can be synthesized by use of a
Ziegler type of stereospecific catalyst. This controls
the position of each monomer unit as it is being polymerized to allow the formation of a regular chain structure from the asymmetric repeating unit.
Polypropylene has many industrial and commercial uses. Since it is resistant to fatigue, plastic hinges, like those on flip-top bottles, are made from polypropylene. It is used for surgical sutures, and it has been used for surgical mesh to repair abdominal and pelvic hernias.
Polyamides (Nylons)
The polyamides represent a synthetic polymer formed by the linkage of a carboxylic acid group [R-COOH] of one molecule and an amino group [NH2] of another:
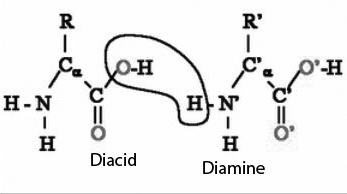
They are designated by the number of carbon atoms in the parent diamine and diacid. Polyamides occur both naturally and synthetically. Natural polyamides are proteins like wool and silk. An important group of synthetic polyamides are the nylons. The polyamides are sometimes also known as nylons, though technically there are many other non-nylon polyamides, such as Kevlar.
Nylons have excellent fiber-forming abilities because of interchain hydrogen bonding and a high degree of crystallinity, which increases strength in the fiber direction.
The basic chemical structure of the repeating unit of
polyamides can be written in two ways (Park, 1994):
-[NH(CH2)xNHCO(CH2)yCO]n
[NH(CH2)xCO]n
Nylons are hygroscopic (absorb water) and lose their strength in vivo when implanted. Proteolytic enzymes may also aid hydrolyzation, and polyamides are readily attacked by strong acids. There are many commercial uses for nylons and other polyamides
Back to Top
Acrylic Polymers
Acrylics are esters of acrylic acid [CH2=CHCO2H]; i.e., they are the products of a reaction of acrylic acid and an alcohol. The basic chemical structure of repeating units of
acrylics is represented by the equation above. Poly(methyl methacrylate), or PMMA, has both R groups as CH3. These polymers are formed by addition polymerization, and they can be obtained in liquid monomer form or as fully polymerized beads, sheets, and rods (Park, 1994).
Acrylic polymers have many medical application, including hard contact lenses, implantable ocular lenses, and bone cement poly(methyl methacrylate) for fixation of joint prostheses. Acrylic polymers have excellent physical properties and are easily colored and easily fabricated. This makes acrylics especially applicable for dentures and maxillofacial prostheses.
Acrylic polymers are
usually obtained in a clear amorphous state. PMMA, for example, has high tensile strength (60 MPa) and softening temperature (125° C). It also has excellent light transparency (92% transmission), a high index of refraction (1.49), and excellent weathering properties. While it is brittle compared to other polymers, PMMA can be cast, molded, or machined with conventional tools. This combined with its excellent chemical resistivity and biocompatibility in pure form makes it a good material for biomedical uses.
Bone cement widely used in orthopedic applications is primarily composed of poly(methyl methacrylate) powder and a monomer methyl methacrylate liquid. Surgical Simplex Radiopaque Bone Cement, a popular commercial product, consists of two packets. One is an ampule of a colorless, flammable liquid monomer that has a sweet, slightly acrid odor with the following composition (Park, 1994):
| Methyl methacrylate (monomer) |
97.4 val% |
| N,N,-dimethyl-p-toluidine Hydroquinone |
2.6 val% |
| Hydroquinone |
75 ± 15 ppm |
The hydroquinone is added to prevent premature polymerization, which may might be triggered by exposure to light, elevated temperature, or radiation. N,N-dimethyl-p-toluidine promotes or accelerate "cold-curing" of the finished compound. "Cold-curing" means the polymerization process occurs at room temperature as opposed to polymerization under high temperature and pressure. The liquid in the ampule is sterilized by membrane filtration.
The second packet is 40 g of finely ground white powder with the following composition (Park, 1994):
Poly(methyl methacrylate) |
15.0 weight % |
Methyl methacrylate-styrine copolymer |
75.0 weight % |
Barium sulfate (BaS04), U.S.P.
|
10.0 weight % |
When the two packets are mixed together, the monomer liquid is polymerized by the free radical (addition) polymerization process producing the "bone cement." The cement viscosity changes over time from a liquid to a dough which can be molded and surgically applied for orthopedic applications as it finally hardens into a solid cement-like material. A minute amount of dibenzoyl peroxide is present in the powder. It acts as an initiator reacting with the monomer to initiate free radical generation with a propagation process that continues until long-chain molecules are produced. Heat is released in this process which must be monitored by the surgical team. It is usually well dissipated by the orthopedic implant and normal tissue blood flow.
Back to Top
|